Headlines
Johnson & Johnson Seeks FDA Emergency Approval for COVID-19 Vaccine
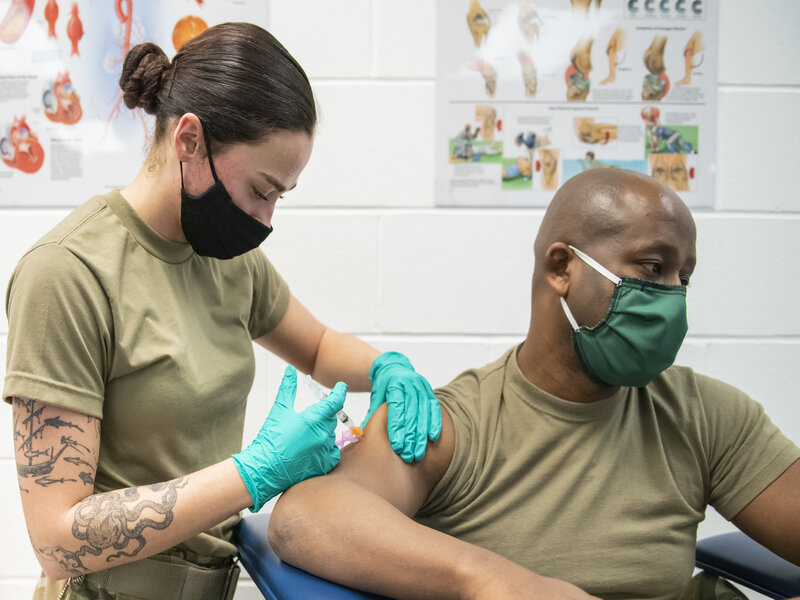
Johnson & Johnson (J&J) recently announced their single-dose Janssen COVID-19 vaccine was ready for use in the United States. On Feb. 4, 2021, the company applied for an emergency use approval from the Food and Drug Administration.
Paul Stoffels, chief scientific officer at J&J said:
Upon authorization of our investigational COVID-19 vaccine for emergency use, we are ready to begin shipping.
With our submission to the FDA and our ongoing reviews with other health agencies around the world, we are working with great urgency to make our investigational vaccine available to the public as quickly as possible.
Acting FDA Commissioner Dr. Janet Woodcock states a meeting has been to discuss the scientific data and information with J&J for February 26. She added that the discussion would help ensure that the public clearly understands their Janssen COVID-19 vaccine.
Both Moderna and Pfizer-BioNTech were granted distribution through an FDA emergency authorization. Their COVID-19 vaccines require two doses and must be kept frozen. Whereas the J&J vaccine only needs to be refrigerated and only requires one shot.
J&J Single-Shot Janssen COVID-19 Vaccine
 When the company signed its $1 billion contact its contract with the U.S. in August 2020, it pledged to have 12 million of its COVID-19 vaccines ready by the end of February 2021.
When the company signed its $1 billion contact its contract with the U.S. in August 2020, it pledged to have 12 million of its COVID-19 vaccines ready by the end of February 2021.
They planned to ramp up delivery to a total of 100 million doses by the end of June 2021, reports The New York Times on January 13.
Operation Warp Speed’s lead manufacturing advisor, Dr. Carlo de Notaristefani, acknowledges the company reported the production delay. He believes Johnson and Johnson might be able to catch up with their original goals by March.
Having a third effective vaccine in the United States will be extremely helpful. The FDA website indicates 89,287 individuals died in December 2020. The U.S. total deaths from COVID-19 is 29.6 million as of February 6 — 426 thousand infections since the beginning of the pandemic nearly a year ago.
As of February 6, 8.7 percent of the U.S. population have been inoculated with one dose, and 2.3 percent have received both required coronavirus vaccine injections. Since J&J Jannsen COVID-19 vaccines only require one dose to be effective, twice as many Americans could be vaccinated.
Written by Cathy Milne-Ware
Sources:
J&J: Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial
C|Net: Johnson & Johnson seeks FDA emergency authorization for COVID-19 vaccine; by Abrar Al-Heeti
The New York Times: Johnson & Johnson Expects Vaccine Results Soon but Lags in Production; by Carl Zimmer, Sharon LaFraniere, and Noah Weiland
Featured and Top Image by Jacqueline Marshall Courtesy of Wyoming National Guard’s Flickr Page – Creative Commons License
Inset Image Courtesy of Jernej Furman’s Flickr Page – Creative Commons License